Research Companion for Medical Device Manufacturers
Conduct and monitor your different trials at the same time, meet your regulatory requirements and collaborate with other researchers with EasyMedStat
Meet Regulatory Requirements
Perform trials while meeting different regulatory requirements such as the MDR and IVDR. EasyMedStat helps you remain compliant at all time
Efficient Trials Throughout The Life-Cycle Development Of Devices
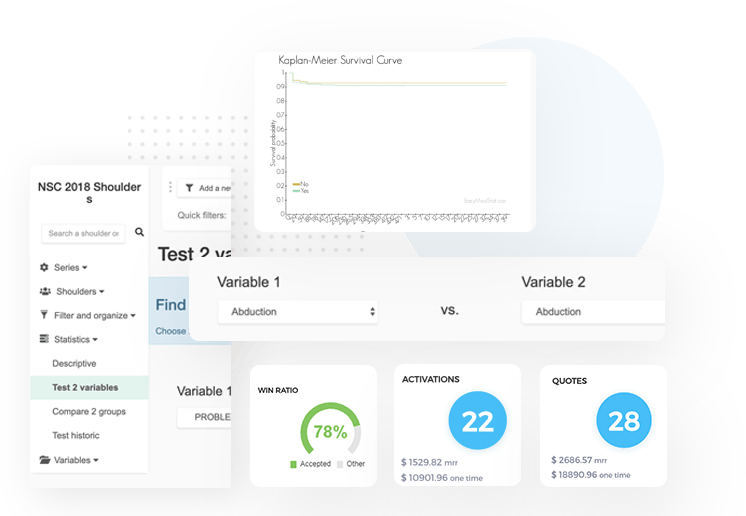
The life-cycle development of your devices is a long and complicated process: however, we help you master the data management and statistics components
Collaborate Easily With Clinicians That Use Your Devices
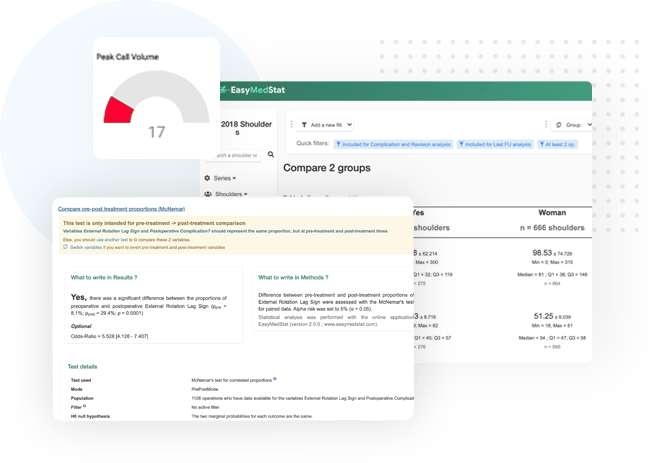
Our platform allows you to have an overview of your study via our user-friendly dashboard. Monitor throughout the research life-cycle with clinicians & researchers and act quickly if needed
An Intuitive User Experience
Get started quickly with EasyMedStat - there's no training required. If you do need help along the way, our dedicated team will step in to give you the support you need
Collaborate With Other Researchers
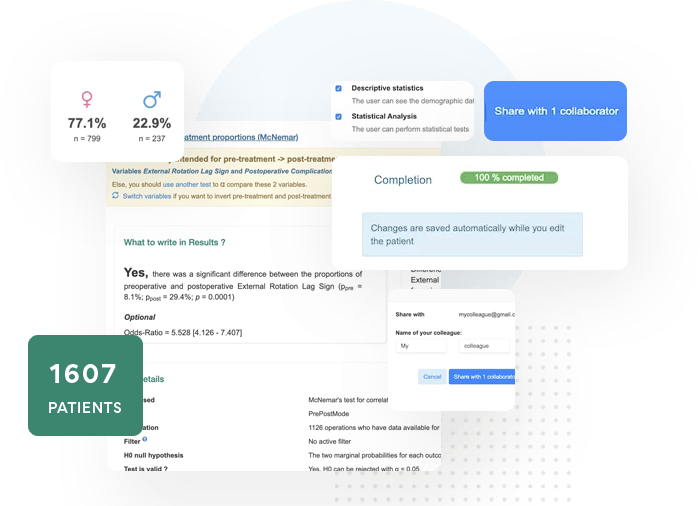
Our tool lets researchers around the world collaborate in a secure environment. Work together on the same database and keep track of your studies with EasyMedStat
A Secure, Legally-Compliant Framework For Your Research
With EasyMedStat, you know your patient data is safe. We leverage pseudonymization, high security standards and compliance with GDPR and CNIL to ensure your work adheres to regulatory and ethical protocols
There's More...